Cadmium is a metallic element that is present in the crust of the Earth and is used in many different sectors, such as batteries and pigments. But using it has significant dangers to your health and the environment. This essay will examine the various aspects of cadmium, its uses, and the difficulties it faces in the modern world.Cadmium is frequently utilized in electroplating and batteries because of its exceptional electrical conductivity. Furthermore, solutions containing cadmium and its derivatives are hazardous and should be handled with caution.
The electronic configuration of cadmium (Cd) is [Kr] 4d¹⁰ 5s².
Or
1s² 2s²2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰5s²
yellow cadmium ( cadmium sulphate CdS)
Cadmium yellow, orange, and red are well-known artist colors that are brilliantly colored, have good persistence, and tinting strength. They are also commonly used as architectural paints because they may give representations more life and vibrancy. Plastics and specialty paints, which need to withstand processing or service temperatures of up to 3,000 °C (5,430 °F), are the main applications for them.Cadmium needs to be protected from air in order to maintain its colorfastness or permanence because the element progressively forms carbonate salts when exposed to it. For fresco or mural painting, cadmium hues will fade, but most paint vehicles achieve this.
Cadmium yellow or cadmium sulfide (CdS).The solid mixture of cadmium selenide and cadmium sulfoselenide is called C.I. Pigment Orange . depending on the ratio of sulfur to selenium.Zinc cadmium sulfide is a solid solution of zinc sulfide and CdS that has a greenish tint.Sometimes cadmium yellow and viridian are combined to create cadmium green, a vivid, light green combination.
In lampworking, borosilicate glass is colored by artists using compounds including cadmium. The palette is distinguished by unusually vivid and saturated tones not present in other colored glass; it is frequently referred to as “cadmium colors” or “cadmium-based colors.”
The history of cadmium pigments used in borosilicate is somewhat recent; Henry Grimmett of Glass Alchemy introduced the first commercial compositions under the name Crayon Colors in 2000.Glass containing cadmium compounds has a limited heat tolerance when melted, so care must be used while working with lamps to prevent the cadmium sulfide from boiling out. With a boiling point of 1,800 °F (980 °C), CdS is a pigment with a maximum temperature tolerance slightly above borosilicate’s operating temperature range.
Industrial use of cadmium
Batteries: Because cadmium can store and release electrical energy effectively, it is frequently used in nickel-cadmium (NiCd) batteries. Power tools, emergency backup systems, and portable gadgets are just a few of the uses for these batteries.
Coatings: Steel and other metals are resistant to corrosion when coated with cadmium, which makes them appropriate for usage in the marine, automotive, and aerospace industries. Electrical component conductivity is improved and corrosion is avoided by cadmium plating.
Semiconductors: Cadmium telluride (CdTe) is a semiconductor material used in thin-film solar cells, photovoltaic panels, and radiation detectors due to its high efficiency and low production costs.
Alloys: Cadmium is sometimes alloyed with other metals, such as copper, to improve their mechanical properties and resistance to corrosion. These cadmium alloys are used in bearings, solder, and low-melting-point alloys for specialized applications.
Health impacts 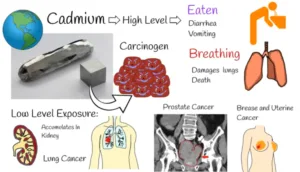
Kidney damage is the main effect that cadmium has on the kidneys. Chronic excessive cadmium exposure can cause renal damage and eventually renal failure. Over time, the accumulation of metal in the kidneys can cause functional impairments, including reduced kidney function in filtering waste materials from the blood.
Damage to Bones: When cadmium replaces calcium in bones, the result is weakening bones and a higher chance of fractures. Osteoporosis can arise from its interference with bone metabolism, particularly in elderly people.
Issues with the Respiratory System: Breathing in dust or vapors containing cadmium can irritate the respiratory system, resulting in emphysema or chronic bronchitis. Lung cancer risk may also rise with prolonged exposure.
Cardiovascular Effects: Research indicates that exposure to cadmium may raise the risk of heart disease and hypertension, among other cardiovascular disorders. Cadmium has the ability to cause inflammation in the cardiovascular system and interfere with blood vessel function.
Reproductive Toxicity: In both males and females, cadmium exposure has been connected to reproductive toxicity. It can result in lower fertility and sperm quality in men, and negative effects on the ovaries and menstrual cycle in women.
Cancer Risk: Prominent health organizations have designated cadmium as a human carcinogen. The chance of developing lung cancer and prostate cancer is increased by prolonged exposure to cadmium.
Environment issues:
Soil Contamination: Industrial processes, agricultural practices, and waste disposal are the main ways that cadmium enters the environment. Cadmium can contaminate agricultural fields and pose dangers to plant health and food safety once it is discharged into the soil, where it can remain for extended periods of time. Elevated cadmium levels in soil can lower crop production and make the area unfit for cultivation.
Water pollution: Mining activities, inappropriate waste product disposal, and industrial discharges can all cause cadmium to contaminate water sources. Water bodies that are contaminated can endanger both human health and aquatic ecosystems. Aquatic species like fish and shellfish can bioaccumulate cadmium, which poses a health risk to consumers.
Airborne Emissions: Cadmium fumes and particles are released into the atmosphere via the burning of fossil fuels, industrial activities, and waste material incineration. Exposure to air pollution including cadmium can be harmful to the health of neighboring communities, especially those residing in urban and industrial regions.
Effect on Wildlife: Ecosystems can be upset and wildlife populations can be harmed by cadmium poisoning. Cadmium exposure can lead to lower survival rates, aberrant development, and reproductive issues in birds, mammals, and aquatic animals. The build-up of cadmium in the food chain can intensify its effects and cause extensive harm to the environment.
Bio Accumulation
Water Contamination:
A variety of sources, such as air deposition, agricultural runoff, and industrial discharges, allow cadmium to reach aquatic habitats. Cadmium can dissolve in water and become accessible for aquatic creatures to absorb.
Cadmium Uptake by Aquatic Organisms:
Cadmium is absorbed from water by aquatic organisms (fish, shellfish, algae) through their gills or by eating contaminated food and debris. Mollusc shells and other tissues such as the liver, kidneys, and gills can all become accumulated with cadmium.
Food Chain Transfer:
Cadmium builds up and concentrates at every trophic level of the food chain when larger creatures eat smaller ones that contain cadmium. Cadmium levels in predatory fish at the top of the aquatic food chain can rise significantly over time.Cadmium is capable of both bioaccumulation within individual species and biomagnification, a process in which the metal’s concentrations dramatically rise as it ascends the food chain. Because of biomagnification, organisms at higher trophic levels may be exposed to larger quantities of cadmium.
Human Exposure: Eating tainted seafood, especially fish and shellfish from cadmium-polluted seas, can expose humans to cadmium. Eating food tainted with cadmium over an extended period of time can cause kidney damage, bone diseases, and a higher risk of cancer, among other health issues.
Japan case study (itai- itai)
With regard to cadmium poisoning and its effects on the environment and human health, Japan has encountered many difficulties, especially when it comes to industrial pollution and agricultural practices. The cadmium pollution of rice fields in the Jinzu River basin of Toyama Prefecture is one famous case study in Japan.The following are some salient features of the Japanese case study on cadmium contamination:
Historical Industrial Pollution:
Mining, metal smelting, and chemical manufacture are just a few of the heavy industries that have historically occupied Japan’s Toyama Prefecture. Historically, these industries have contaminated the area’s land, water, and air by releasing cadmium and other pollutants into the ecosystem.
Rice farming and cadmium:
It is well known that cadmium builds up in rice fields, especially in areas where the soil has significant concentrations of cadmium. Due to cadmium uptake by rice plants and subsequent human exposure from eating contaminated rice, cadmium poisoning of rice fields in Toyama Prefecture has become a major concern.
Effects on Health:
Prolonged exposure to rice tainted with cadmium can cause major health issues such as kidney damage, bone abnormalities, and a higher chance of developing cancer. Cadmium exposure posed health dangers to residents of affected areas, especially those who depend on locally cultivated rice as a primary meal.
Government Reaction: To address the issue of cadmium contamination in Toyama Prefecture and other affected locations, the Japanese government implemented a number of actions. These precautions included monitoring the levels of cadmium in rice and other agricultural products, limiting the growing of rice in hazardous regions, and improving the soil.
Raising Awareness and Advocating for Stricter Regulations and Pollution Control Measures: Local communities and environmental organizations were instrumental in bringing cadmium poisoning to the public’s attention. The government was compelled to act to address the problem and safeguard public health as a result of public pressure and community involvement.
How to mitigate cadmium pollution
A multifaceted strategy is used to mitigate cadmium contamination with the goal of lowering environmental exposure and decreasing health concerns. The following are some essential tactics for reducing cadmium pollution:
contamination Prevention: It’s critical to put policies in place to stop cadmium contamination before it starts. This entails controlling wastewater treatment requirements, limiting the amount of cadmium released into the environment through the promotion of cleaner production methods, and regulating industrial emissions.
Soil Remediation: Remediation methods that help lower cadmium levels in polluted soil include soil washing, phytoremediation (using plants to absorb and detoxify pollutants), and soil additives. By immobilizing or eliminating cadmium from the soil, these techniques seek to reduce the amount of cadmium that plants can absorb.
Waste Management: Cadmium can be kept out of soil and water systems by managing industrial waste, electronic trash (e-waste), and agricultural runoff properly. Adverse environmental exposure to cadmium can be reduced through recycling and appropriate disposal of products containing the metal.
farming Techniques: Using sustainable farming techniques can aid in lowering the amount of cadmium that accumulates in crops. This covers crop rotation, controlling the pH of the soil, and using organic fertilizers devoid of cadmium.
Monitoring and Regulation: To pinpoint contamination hotspots and evaluate the hazards to the public’s health, it is imperative to regularly measure the amounts of cadmium in soil, water, and food products. Standards for cadmium levels in food, water, and air are largely set and enforced by governments and regulatory bodies.
Public Education and Awareness: Educating the public about the health concerns of cadmium exposure and encouraging proper handling and disposal methods will enable people to take action to reduce cadmium pollution on a personal and community level.
Research and Innovation: New approaches to mitigating cadmium pollution can be sparked by ongoing investigations into cleaner production techniques, substitute materials, and remediation technology. Long-term environmental conservation requires funding for sustainable solution creation and research.
Reaction with other metals
Depending on the particular circumstances and the chemical characteristics of the metals involved, cadmium exhibits a variety of interactions with other metals. Cadmium is frequently involved in the following reactions:
Cadmium is a metal that easily forms alloys with a variety of other metals, such as zinc, copper, and silver. For instance, tiny amounts of cadmium may be added to brass, an alloy of copper and zinc, to improve its low-friction and corrosion-resistant qualities.
Galvanic corrosion: This type of corrosion can happen when cadmium comes into contact with some metals in the presence of an electrolyte, like water or an acidic solution. Because cadmium is more reactive than some other metals, such as steel or iron, it can serve as a sacrificial anode by corroding one metal more than another in order to shield the other from corrosion.
Cadmium electroplating A typical industrial procedure called electroplating is used to apply a thin layer of cadmium on steel, iron, and other metals to prevent corrosion. Cadmium ions in solution are lowered and deposited onto the metal substrate’s surface during the electroplating process, creating a protective layer.
Redox Reactions: Depending on the circumstances of the reaction, cadmium can act as an oxidizing or reducing agent in redox reactions with other metals. For instance, cadmium metal can be produced by oxidizing chemicals reacting with cadmium oxide (CdO), and vice versa when oxidizing agents are present.
Formation of Complexes: Cadmium ions have the ability to form complexes in aqueous solutions with a variety of ligands, such as organic compounds and water molecules. In comparison to free cadmium ions, these complexes might have distinct chemical characteristics and solubilities that impact how cadmium behaves in solution and interacts with other chemicals.
conclusion
In summary, cadmium poisoning poses serious risks to both environmental sustainability and human health. The detrimental impacts of exposure to cadmium, such as increased cancer risk, bone diseases, and kidney damage, highlight how urgent mitigation measures are. Cadmium contamination can be decreased and ecosystems and populations shielded from its damaging effects by enforcing regulatory requirements, encouraging sustainable farming practices, and putting pollution control measures into action.


Thanks for sharing. I read many of your blog posts, cool, your blog is very good.