
Understanding acid rain
The acid is a great concern for the future agriculture practice,as it poses significant threat to ecosystem, agriculture and marine life. This phenomenon occurs due the oxides of sulphur (so2)and nitrogen N2O . These oxides are mainly produced by the combustion of fossil fuels like coal and petroleum
According to estimates by association for emission of greenhouse gases from federal land
| Source | CO2 emissions MMTco2Eq | CH4 emission MMT co2Eq | NO2 MMT co2 Eq |
| Coal | 725.36 | 2.09 | 3.68 |
| Motor gasoline | 110.892 | 0.143 | 1.239 |
| Coal (individual) | 9.3 | 0.268 | 0.047 |
These oxides are swept up in the atmosphere and can travel upto 1000 kilometres. The longer they stay in the atmosphere are more likely to get oxidised in acids or can even cause smog.when these oxides react with the water (vapour form) in the atmosphere can result in formation of sulphuric acid H2SO4 and Nitric acid HNO3 and fall in the group in the form of acid rain or may stay in the atmosphere and can cause fog or smog.
Impact on soil
The acid rain mainly impact the PH of soil and the soil becomes more acidic . This acidity can affect the soil structure, nutrition availability and microbial activity which plays a pivotal roles for the growth of plants.

Crop production: the Acidic rain usually responsible in degradation and nutrition deficiencies of soil and so it will become infertile for the production and crop yield.This worst level of pollution is not only impacting the plants but also the livelihood of farmers. Moreover the degradation our fosters caused by acid rain cause the wild animals to migrate are either lead into wild conflicts. So they are also facing stress upcoming in danger and even can lead to cause extinct.
Metal toxicity: As the pH of soil decreases aluminium an naturally occurring metal in the soil become more soluble and toxic to plant life as it impacts root development of plants in the soil which directly affected the growth of plants.

Aquatic life : The acid rain have a direct impact an aquatic life. As the pH of water increases it becomes unfit far aquatic life
- Reproduction and growth:The acidification can cause interference with reproductive cycle example, the high acidic water impacted the development of fish egg which results the reduction of prediction of aquatic growth
- Food chain:It was found the acidification off water have disrupted the symbiotic relation how many species and can reduce the availability of food for other aquatic forms. Acid rain has direct or indirect impact on other food chains of marine life as well. From last decade we have seen an abrupt reduction production of fish in many water bodies all over the world
- Non living matter: The Taj Mahal in India is losing it is beauty do too marble of leprosy caused by acid rain number second The British parliament building also support add image due to acid rain

-
Acid rain control programme
- The Acid Rain Program (ARP), the main moto of acid rain control programme forces the industry significantly reduce its emissions of nitrogen oxides (NOx) and sulfur dioxide (SO2), which are the main precursors of acid rain. The total amount of SO2 that electric generating units (EGUs) in the contiguous United States are permitted to emit is permanently capped under the SO2 program. The scheme was implemented gradually, with an ultimate 2010 SO2 cap of 8.95 million tons—roughly half of the power sector’s 1980 emissions—set. Under the ARP, NOx reductions are accomplished by a program that is more akin to a conventional, rate-based regulatory system and is applicable to a portion of coal-fired EGUs. Since the start of the programme
The ARP has significantly reduced emissions since it started in 1995. For additional details on the ARP’s development, view our yearly progress reports.
The ARP established a system of allowance trading that employs market-based incentives to reduce pollution. It was the nation’s first nationwide cap and trade program. It has shown to be a very successful method of achieving emission reductions, meeting environmental goals, and improving human health to cut emissions via a market-based system, which gives regulated sources the freedom to choose the most economical method.
history
Following World War II, countries in Europe and eastern North America experienced a significant increase in their fossil fuel use, which led to the emergence of modern anthropogenic acid deposition in those regions. The 1972 United Nations Conference on the Human Environment in Stockholm, Sweden, marked the beginning of international cooperation to address air pollution and acid deposition. The framework for lowering acid deposition and air pollution in Europe was established in 1979 by the Geneva Convention on Long-range Transboundary Air Pollution. The convention resulted in the first international accord with legal force to lower air pollution on a large regional scale. Since its original conception, this first agreement has been expanded by other methods.
The United States has experienced a decrease in acid deposition as a result of the Clean Air Act of 1970 and its 1990 revisions. In the 1970s, efforts were made to draft a Memorandum of Intent between the United States and Canada to lessen acid deposition and air pollution. But it wasn’t formally established until the 1991 Canada-US Air Quality Agreement, which set long-term limits on SO2 emissions and provided guidelines for lowering NOx emissions in both nations.
After reaching their peak in the late 1970s, SO2 emissions in the US and Canada started to decrease as a result of government-mandated air pollution regulations. Beginning in 1995, the U.S. Clean Air Act Amendments of 1990 mandated the first phase of emission reductions.primarily through the control of emissions from coal-fired power plants. With this achievement, the United States started implementing additional major reductions in SO2 emissions, which led to an 88 percent decrease in SO2 emissions between 1990 and 2017.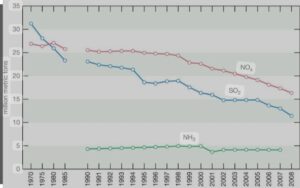
On the other hand, NOx emissions in the US peaked around 1980 and were largely steady until the end of the 1990s,when restrictions on emissions from cars and power plants caused emissions to start to significantly fall. Since around 1980, NOx emissions have surpassed SO2 emissions; however, since the Clean Air Act went into effect, SO2 emissions have also decreased.
For instance, NO2 emissions decreased by 50% between 1990 and 2017. Sulfate (SO42) and nitrate (NO3) deposition, as well as acid deposition, significantly decreased during this time due to the combined reductions of SO2 and NOx emissions. Some areas of the United States continue to see increases in ammonia (NH3) and ammonium deposition, particularly in areas with intense livestock and agricultural activity.
Acid deposition has been greatly decreased in both eastern North America and Europe as a result of agreements and initiatives like the ones mentioned above. From the mid-1960s through 2016, the H+ concentration in precipitation decreased by approximately 86% at the Hubbard Brook Experimental Forest in New Hampshire, the United States, which has the longest continuous record of precipitation chemistry in North America.
Data from measuring stations spread around the eastern United States also showed similar patterns, with measurements showing a roughly 40% drop in H+ concentration between 1994 and 2008. The average yearly SO2 and nitrogen concentrations found in both wet and dry acid deposition declined significantly throughout the eastern United States between, according to EPA monitoring stations, which are primarily located in urban areas.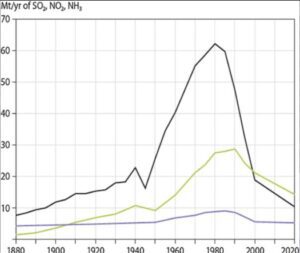
Certain ecosystems damaged by acid deposition in Europe and North America have taken a long time to recover, even with notable drops in acid deposition. The ability of soils to neutralize acid has been diminished in these vulnerable areas due to decades of acid deposition. Because of this, these soils—even at lower levels—are even more vulnerable to ongoing acid deposition. Such acid-sensitive ecosystems will require additional reductions in NOx and SO2 emissions.

